
|
June 2004
 Diacetylene |
|
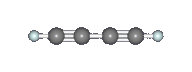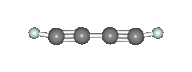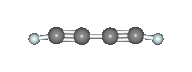
Diacetylene (C4H2) or butadiyne is a linear chain molecule with two C≡C triple bonds. It was identified by Cernicharo et al. in the proto-planetary nebula CRL 618 with the Infrared Space Observatory by means of one of its characteristic vibrational motions, the symmetric C≡C–H bending mode at 15.9 μm (630 cm–1) as seen in the image above. There are two other notable IR features: the C≡C–C≡C bending mode (231 cm–1), and the asymmetric C–H stretching mode (3329 cm–1) (see images below). Diacetylene has also been observed in all four of the gas giant planets, Saturn, Jupiter, Uranus, and Neptune, as well as in the atmosphere of Saturn's moon Titan by Voyager 1. In Titan's atmosphere, diacetylene is mostly likely formed by the reaction between acetylene (C2H2) and the ethynyl radical (C2H), which is produced when acetylene is photolyzed by solar UV radiation in the upper atmosphere. The ethynyl radical can attack C=C double and C≡C triple bonds and react efficiently even at very cold temperatures. |



|
|