
| ||
|---|---|---|
| PREVIOUS SECTION | CURRENT SECTION | NEXT SECTION |
| 7. Atomic Excited States | 8. Ion Energetics | 9. Putting Atoms Together |
| 8. Ionization Energies and Electron Affinities | ||
|---|---|---|
|
In this chapter we built atoms electron-by-electron in some cases. We found that all elements can hold onto at as many electrons are there are protons in its nucleus. If there are Z protons and Z electrons, one has the neutral atom. In most cases, we found that elements can hold onto one more electron than Z to yield the atom's anion. There were some exceptions as well. The rare gases He, Ne, and Ar do not have anions because they cannot hold onto the extra electron. Be and Mg are also closed shell (have no singly occupied orbitals) and won't bind an extra electron. The other oddball in N, which likewise cannot hold onto an extra electron. | ||
|
The neutral atoms are used as an energetic reference. Removing one or more electrons from an atom is call ionization. Removing the first electron from an atom is known as it first Ionization Energy (the term Ionization Potential has also been used extensively). In principle, there are as many ionization energies as there are electrons in an atom (but it can be very difficult to remove all of the electrons from a heavier nucleus). The figure below shows the energy diagram for F. | ||
8.1 | 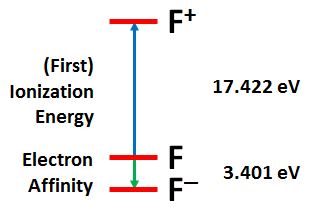
| |
|
It takes 17.422 eV (electron volts) to remove the first electron from F to yield F+. The second, third, and fourth ionization energies of F to yield F+2, F+3, and F+4 are 34.970, 62.707, and 87.138 eV. They become larger and larger. The figure shows another energy difference for F, the energy gained by adding an electron to form F–. This quantity is called the electron affinity of the atom or element. Forming F– from F yields is 3.401 eV. Electron affinities are always much smaller than first ionization energies. | ||
|
The following table shows the first ionization energies for the elements H through Ar. | ||
8.2 | 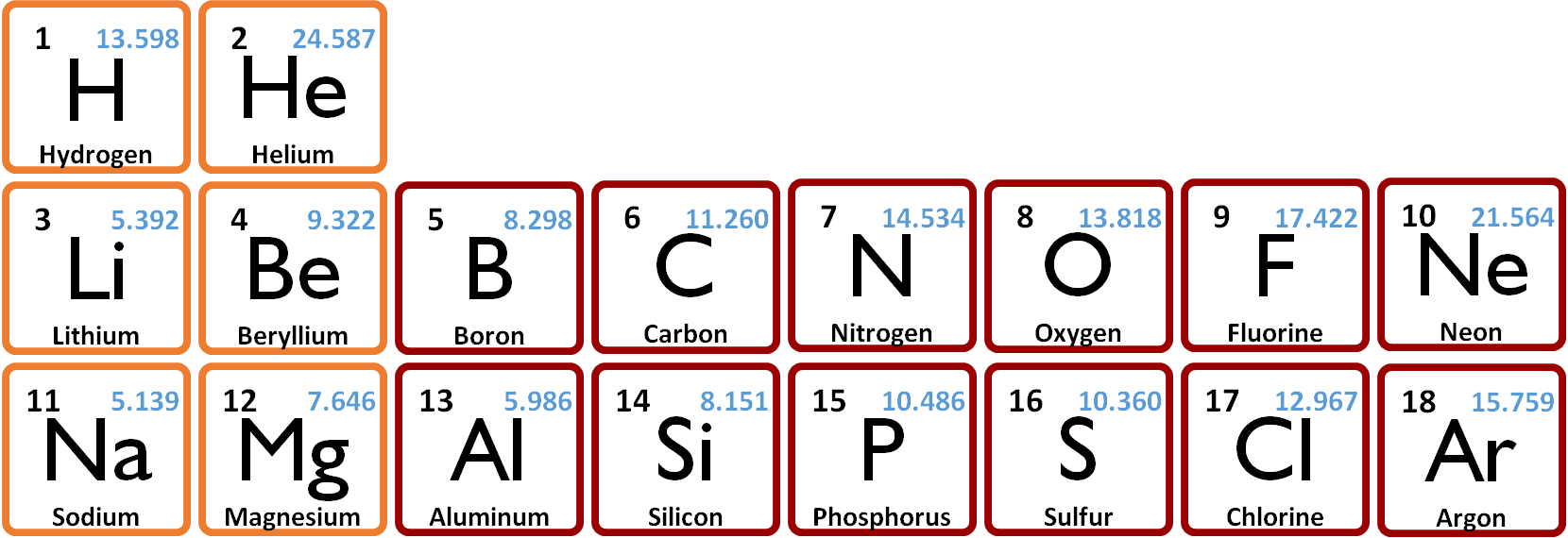
| |
|
For a given row of the periodic table, the ionization energies mostly increase from left to right, with N and P exceptions to the rule. Ionization energies decrease as one goes down columns of the periodic table. | ||
|
The next table shows the electron affinities for H through Ar. Note the zero values for the six elements discussed above (He, Ne, Ar, Be, Mg, and N). | ||
8.3 | 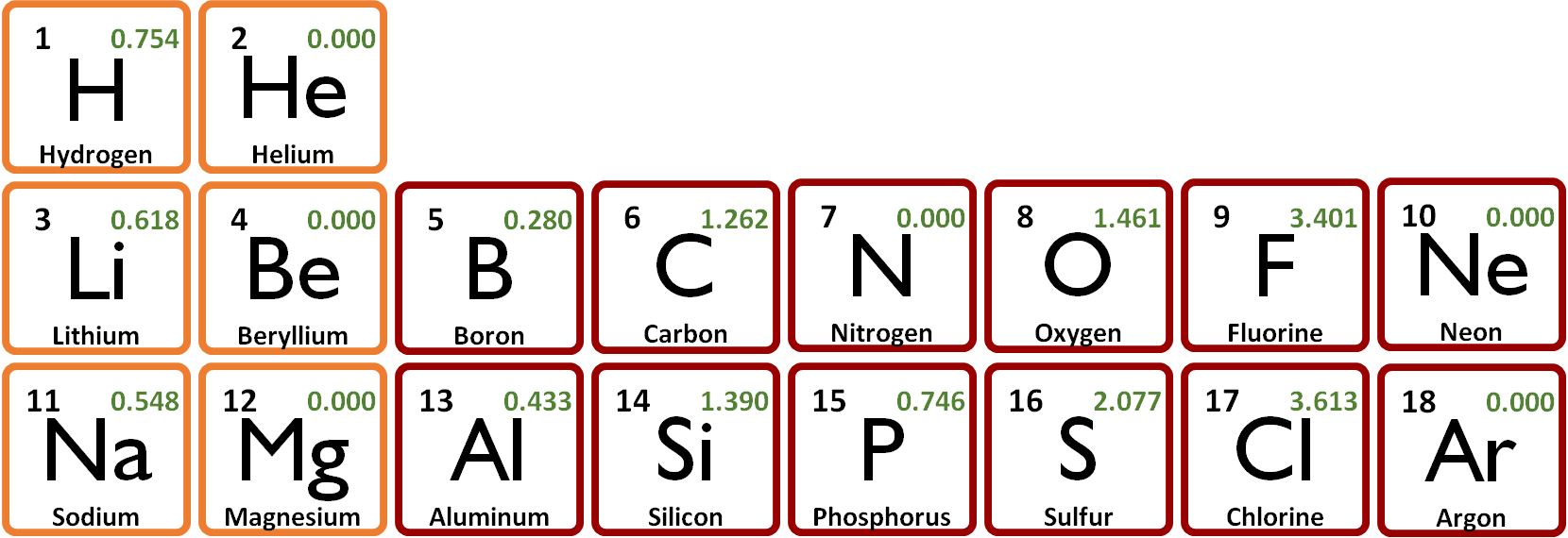
| |
|
Electron affinities also generally increase from left to right across a row (with more exceptions than they are for ionization energies). The values increase moving down columns, except for the alkalais in the first column. | ||
| Click on the link to proceed to the next section: | Chapter 2 – Bonding |
|---|---|
| MODULE TABLE OF CONTENTS | |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|