 
| ||||

|

|

|

|

|
|
MAR 2017/DEW MORE BAD ATOMS. Some time ago I ran across this depiction
of an atom:
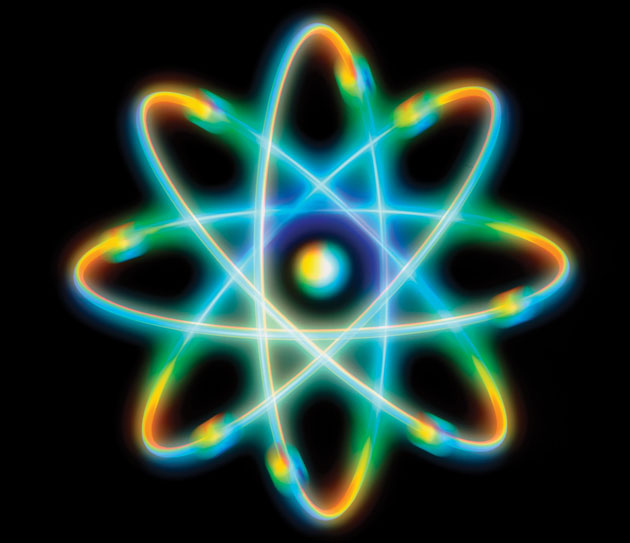 The image is associated with an otherwise reasonable article by Eric Scerri called The Trouble with the Aufbau Principle published in 2013 in Education in Chemistry, a journal from the Royal Society of Chemistry. Here's a link for the story. It's quite possible the graphic was chosen by someone who is not a trained chemist. With its four equivalent classical particles, it's certainly not an accurate representation of a beryllium atom! The webpage above credits the Science Photo Library as the source of the image. Looking over
that archive, it's easy to find more terrible depictions of atoms. Here's another graphic labeled as a beryllium
atom:
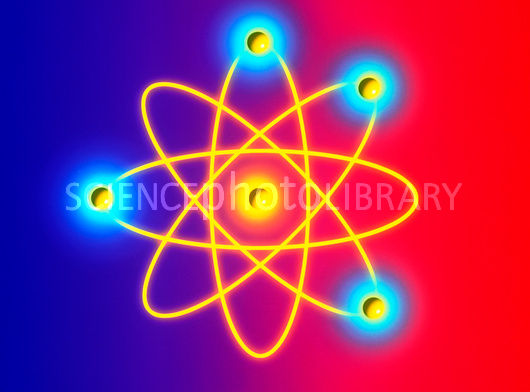 The webpage for the graphic has a caption that is partly accurate but partly egregiously wrong:
"....as it is now thought"??? As if Schrödinger's 90 year-old work and countless subsequent treatments of atomic structure are part of an unresolved controversy? !!! | ||
|---|---|---|
|
FEB 2017/DEW BAD ATOMS & MOLECULES. It is very easy to find examples
of atoms and molecules on the internet that do not reflect quantum chemical reality. Here is one of my favorite
examples (SOURCE):
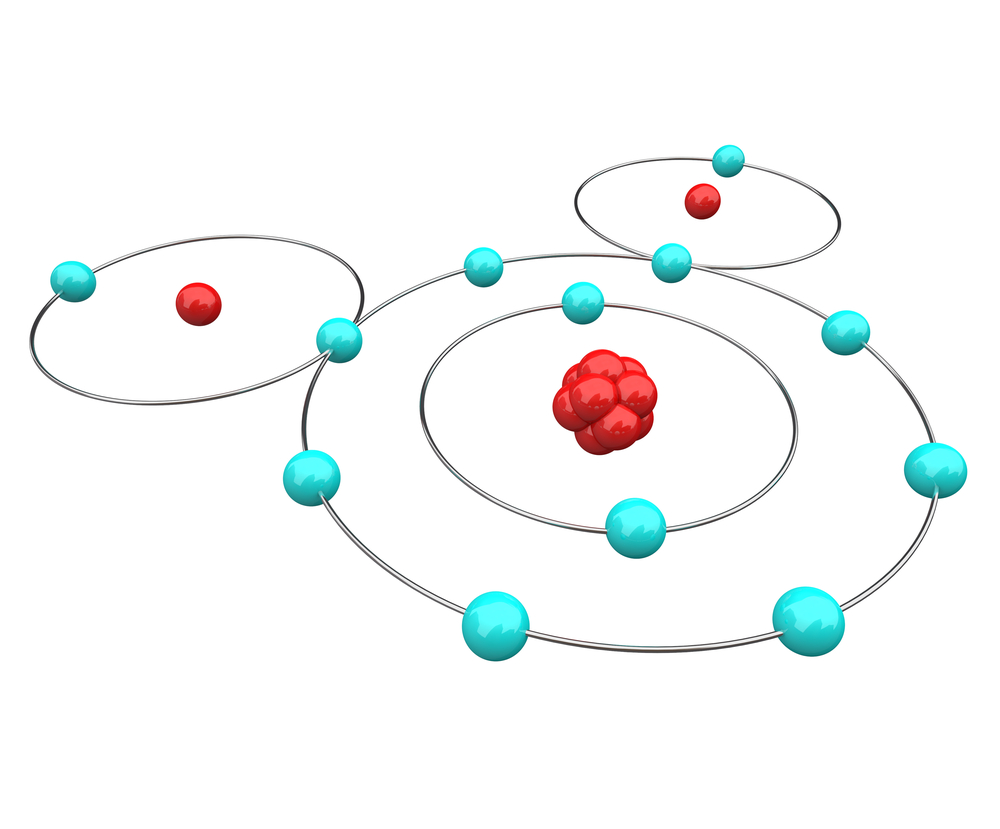 It's quite the clockwork depiction. Electrons (vastly oversized & non-quantum) rest, unmoving and perfectly spaced, in circular rings around the nuclei (again, vastly oversized & non-quantum). Everything lies in the plane containing the nuclei. The rings are coupled together through the two shared electrons, which can't move or they will no longer be shared. The non-shared electrons on the hydrogen atoms sit exactly on the far size from the shared electrons. Finally, there are twelve electrons, not the ten there should be! (I missed that one for several years.) The reality is that Lewis' original conception of atoms and molecules was every bit as bad. He
really thought the electrons were fixed at the corners of cubes, like this
(SOURCE):
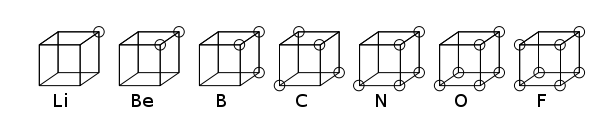 By combining his cubes, he intuited that electrons make pairs in bonds, but his reasoning was
based on such inadequate assumptions that it doesn't seem like it should really count as discovering something
very fundamental about the nature of bonding. Here is the formation of CO2 from Lewis cubes:
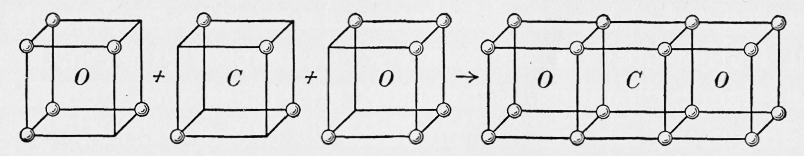 This image was copied from a book intended for introductory college chemistry published in 1924, post-Lewis, of course, but also pre-Schrödinger (An Elementary Study of Chemistry by W. McPherson and W. E. Henderson, Ginn and Company, Boston). While it gets the geometry and double bonding correct, one has to ignore that Lewis believed the positions of the electrons were static, fixed at the vertices of his cubes. In case one is inclined to give Lewis too much benefit-of-the-doubt, this article notes that Earnshaw has demonstrated in 1839 that static arrangements of particles could not be stable (for central forces like Coulombic attraction and repulsion). | ||
|
FEB 2017/DEW My plan to maintain a blog did not pan out. The project has run its course, and I'm wrapping up the development of the WebBook and various publications about the module. We did test the material in a couple ways, but not by incorporating it directly within a course. In December 2014, I presented three lectures about the material to about a dozen grad students in the department and got some good feedback from them. Those lectures are available under Resources. In Spring 2016, we tested a 75 minute version of the module on a group of 60 students who had already passed CHEM 102, the department's first semester non-majors Gen Chem course. The results of this Treatment group were contrasted with results from a Control group of students (n=~780 students) who were taking CHEM 102 that semester. The Treatment group did slightly better on our assessment instrument than the Control group overall, and far better on a subset of the questions that best focused on core chemical concepts. The lecture I presented to the Treatment group is also available under Resources. Eventually, links to the publications about the module and the assessment thereof will be available under Resources. | ||
|
AUG 2011/DEW With this posting, I begin my online journal describing the construction of our NSF-funded module on atoms, bonding, and molecules, which we call "Molecules; An Atom-By-Atom Approach." One of our objectives is to give people a glimpse behind the scenes and provide a forum to explain why we're doing things in certain ways. Why did we embark on this project? With so many general chemistry textbooks already available, what do we hope to accomplish that they have not? The answer is very simple and may, unfortunately, ruffle some feathers: there are a number of problems with the existing approach to teaching the subject. Some of its shortcomings are widely known and recognized, others less so. An understanding of how atoms interact to form molecules and matter is absolutely fundamantal to Chemistry and related disciplines that depend on sound underpinnings in basic Chemistry, such as Biochemistry and Materials Science. It makes little sense to continue to use an inadequate approach when there is a better approach that does not sacrifice rigor and is (we believe) ultimately more intuitively understandable. Our task is to produce the module, assess it in the classroom, refine it as necessary, and disseminate it throughout the Chemistry community. We're eager to receive feedback from people who look at the website. Although this is not a traditional highly interactive blog, I will post comments people send to me—as long as they are apropos, constructive, and civil. I prefer to post non-anonymous comments. |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|