
| ||
|---|---|---|
| PREVIOUS SECTION | CURRENT SECTION | NEXT SECTION |
| 18. Atom-by-Atom Approach | 19. HnO Compounds | 20. HnO+/HnO– |
| 19. HnO Compounds | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Nominal valence and nominal bond angles. Valence is a measure of the maximum number of bonds that can be formed between a given atom and other atoms. The atom in question may well be found in compounds where it does not have the maximum number of bonds. Those compounds are very important in chemistry. Sometimes it is fairly easy to identify an element's valence, and sometimes there are surprises. | ||||||||||||
|
It's useful to think of something we call the nominal valence. It's a simple count of electrons likely to participate in bondind for an atom/element. Here are our diagrams for O: | ||||||||||||
19.1 | 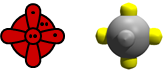
| |||||||||||
| ||||||||||||
|
An O atom has two singly occupied 2p orbitals, so it has a nominal valence of 2, answer (b). Thus we expect the largest molecules that we can build by adding H atoms to one O atom to be H2O. | ||||||||||||
|
The nominal bond angles of an atom are the angles between the singly occupied orbitals when used to form single bonds. | ||||||||||||
| ||||||||||||
|
The angle between the two singly occupied 2p orbitals of O is 90°, so the nominal bond angle of H2O is also 90°, answer (d). | 
| |||||||||||
|
Now that we have some sense of what to expect when we add H to O, let's explore what actually happens and see how well our guesses work out. | ||||||||||||
|
Adding the first H to O. We looked at covalent bond formation between O and H briefly in Section 12 and showed the bond pair orbitals in Figure 12.9. The OH radical is often called the hydroxyl radical. Here we will look at the interaction of O and H more closely. For example, we didn't look at all of the options for H approaching an O atom. Here are the 3D diagrams that cover the possibilities: | ||||||||||||
19.2 | 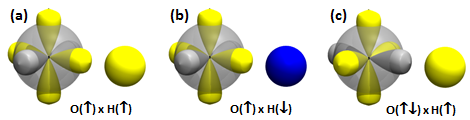
| |||||||||||
| ||||||||||||
|
Here is a graph showing the potential energy curves for the three diagrams: | ||||||||||||
19.3 | 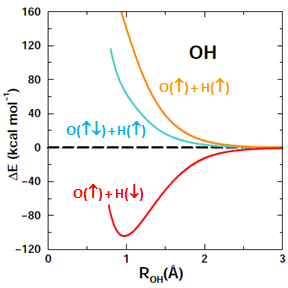
| |||||||||||
|
The correct answer to the question above is (b): OH behaves very much like HF. A covalent bond forms if the spins of the electrons in the O 2p1 and H 1s1 orbitals are antiparallel. No recoupled pair bond forms if H approaches the 2p2 pair of F. Ground state O atom does not have any unoccupied 2p orbitals, so it does not have a lobe pair (as Be, B, and C do). | ||||||||||||
|
Let's look at animations of the bond orbitals for OH formation: | ||||||||||||
19.3 | 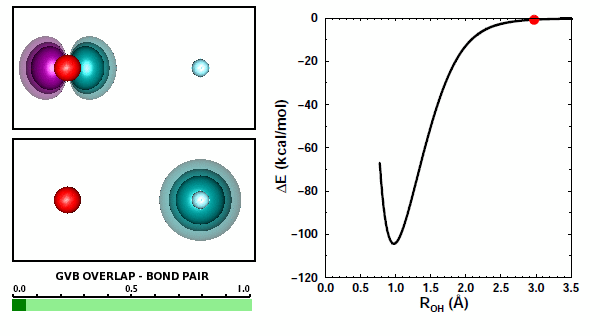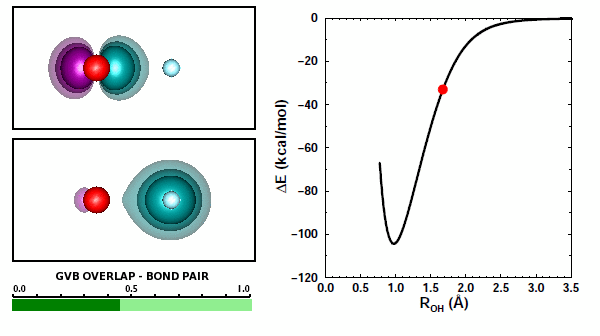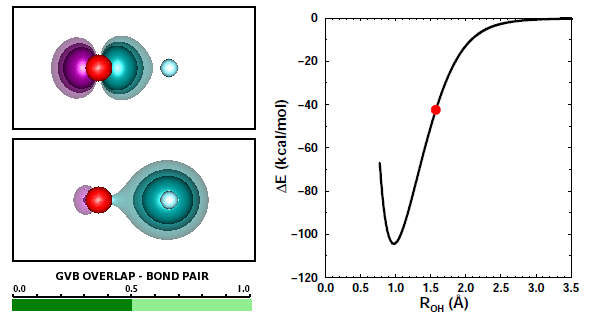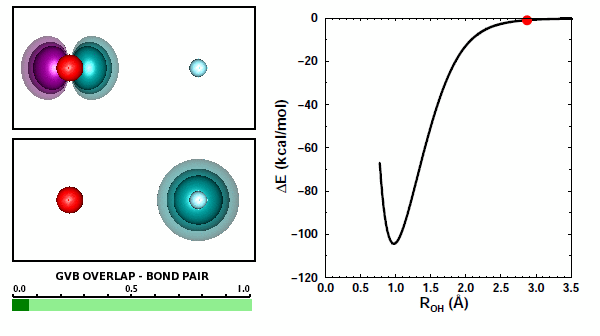
| |||||||||||
|
The animations depict very typical polar covalent bond formation. The H orbitals shifts more toward O than the O orbital shifts toward H, so the net polarization is toward O. As we saw in Section 12, the 2D and 3D bonding diagrams for OH are: | ||||||||||||
19.4 | 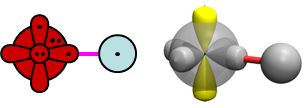
| |||||||||||
|
Adding the second H to OH. If we bring in a second H to the bonding diagrams for OH, we would expect to form a covalent bond with the remaining singly occupied O 2p1 orbital, as long as the spins of the O and H orbitals are suitable. That would give us the following bonding diagrams for H2O: | ||||||||||||
19.5 | 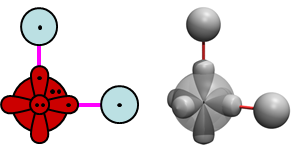
| 
| ||||||||||
|
These diagrams show the nominal 90° bond angle of H2. If we calculate the structure, we can start with 90° and let the geometry relax. An optimum structure for H2 looks like this: | ||||||||||||
19.6 | 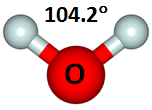
| |||||||||||
|
The optimum angle is about 104°, or 14° larger than the nominal bond angle. | ||||||||||||
| ||||||||||||
|
The best answer here is (b), that the two bond pairs repel each other. There's some truth to answer (c) as well, however, because of the bond polarization in each of the OH bonds of H2O. As we noted above, the bond in OH is polarized toward O. Let's look at the orbital animations for forming the second OH bond in H2O: | ||||||||||||
19.7 | 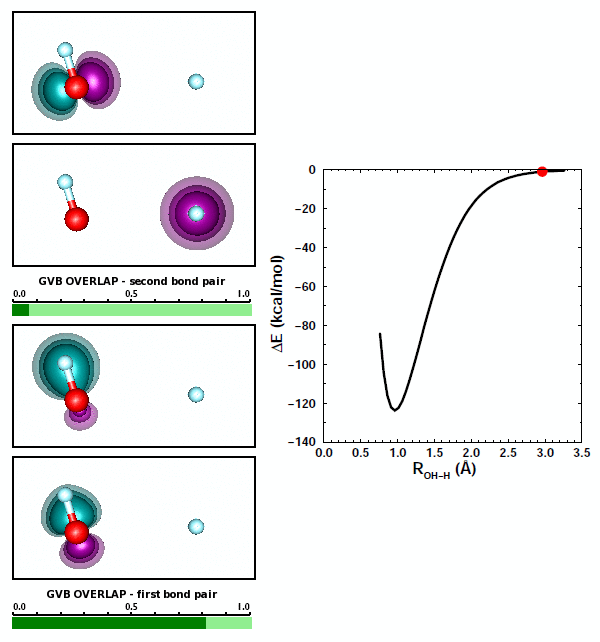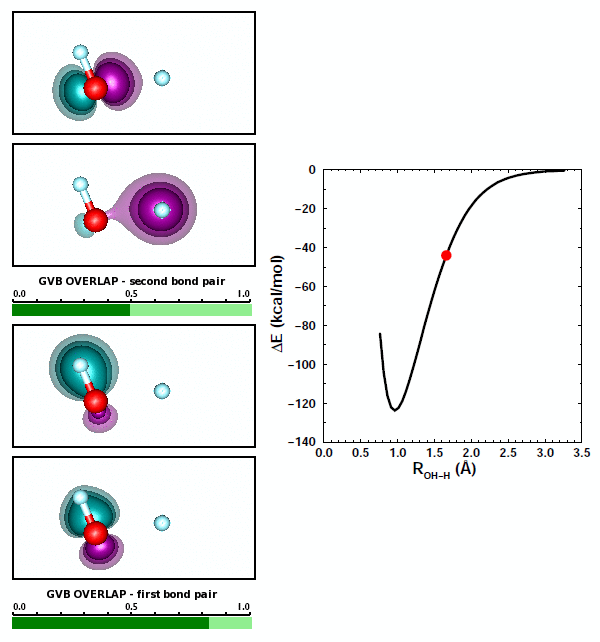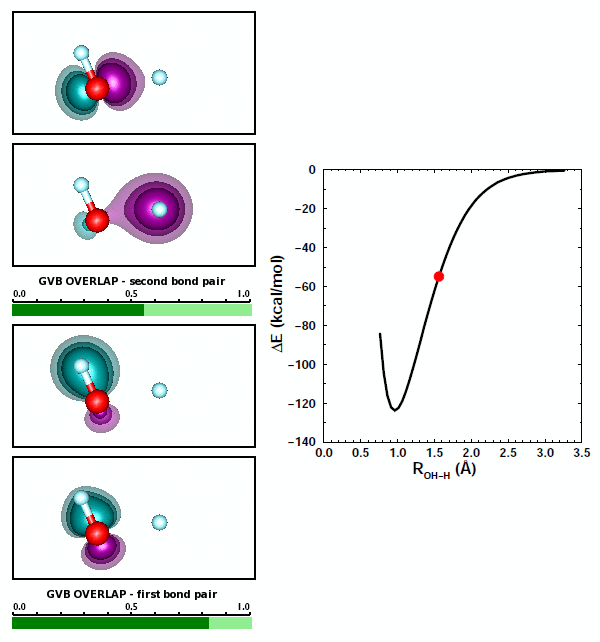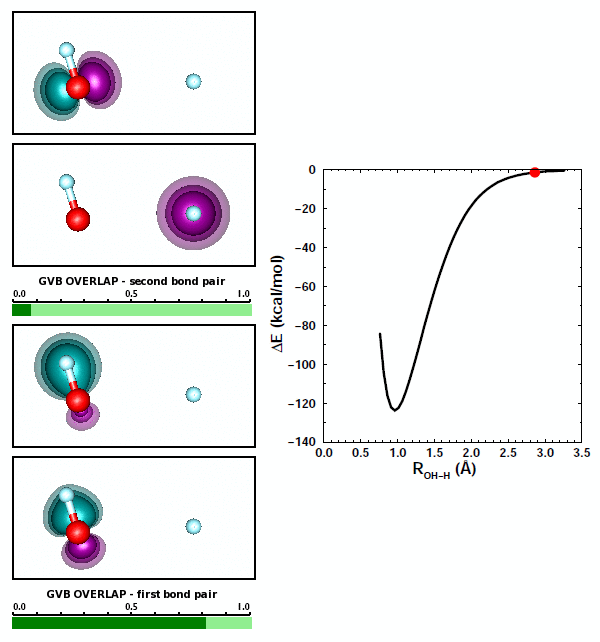
| |||||||||||
|
We can note a couple things about this animation. First, the formation of the second polar covalent bond is very similar to the formation of the first bond: the polarization is also toward O. Second, the orbitals for the first bond change very little in response to the formation of the second bond. | ||||||||||||
|
Both OH bonds are polarized toward O. As a consequence, both H atoms are left with some amount of positive charge. Based on Coulomb's Law, the two H atoms repel each other, adding to the repulsion between the two bond pairs. That maked answer (c) above partly correct. | ||||||||||||
|
Adding a third H to H2O. We can try adding another H atom to water. Look at the bonding diagrams in Figure 19.5 and try to answer the question. | ||||||||||||
| ||||||||||||
|
The fact that we already saw that H cannot form a recoupled pair bond with the O 2p2 orbital is pretty good evidence that it cannot do so in H2O either. Calculations show that H cannot be added to H2O, so answer (b) is the correct one. | ||||||||||||
|
SUMMARY. We've now seen that we can add two H atoms to O to form OH and then H2O. We cannot add a third H atom. That means that the nominal valence of O is the true valence of O. We saw that the nominal bond angle slightly underestimated the real bond angle of H2O, but it was a reasonable starting guess. We understand why the real bond angle is larger than the nominal value. | ||||||||||||
|
The O atom and OH radical are far more reactive than H2O. It's straightforward to understand why: O and OH each have at least one single occupied orbital and can thus form new bonds with H (or other atoms or molecules with at least one unpaired electron), while H2O has no singly occupied orbitals. For something to react to with H2O, it has to disrupt one of the OH bonds. | ||||||||||||
|
Now that we've explored adding H to O, let's see what happens when we add H to two related ions, O+ and O–. | ||||||||||||
| Click on the link to proceed to the next section: | 20. HnO+ and HnO– Compounds |
|---|---|
| MODULE TABLE OF CONTENTS | |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|