
| ||
|---|---|---|
| PREVIOUS SECTION | CURRENT SECTION | NEXT SECTION |
| 19. HnO Compounds | 20. HnO+/HnO– | 21. HnS Compounds |
| 20. HnO+/HnO– Compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
In Section 19 we found two H atoms can be added to the O atom to form the hydroxyl radical (OH) and water (H2O). In this section we will study what happens if we add H atoms to the cation (O+) and anion (O–) of O. Here are the diagrams for them as well as for O: | ||||||||||
20.1 | 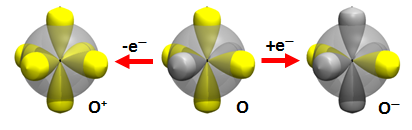
| |||||||||
|
Removing an electron from O yields O+: the electron is taken from the doubly occupied 2p orbital. Thus O+ has the same orbital diagram as N. Likewise, when an electron is added to O to yield O–, the diagram is the same as F. | ||||||||||
|
Since we are interested in comparing behavior across the entire family of HnOp compounds for p={–1,0,1}, here are the structures and sequential bond energies of OH and H2O for reference: | ||||||||||
20.2 | 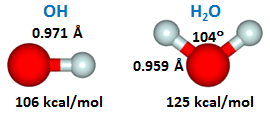
| 
| ||||||||
|
Adding H to O–. We'll start with the O anion. As in O, we begin by asking a fundamental question: | ||||||||||
| ||||||||||
|
Since O– has one singly occupied orbital, its nominal valence is 1, answer (c). We thus expect to only be able to add one H to O– to yield OH–. This is indeed the case. Below is the bonding diagram for OH–, which is known as the hydroxide ion. | ||||||||||
20.3 | 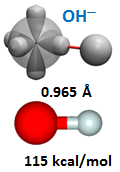
| |||||||||
|
The optimized structure is also shown. Note that the bond length is very similar but slighter less than that of OH, while the bond energy is about 10 kcal/mol greater. | ||||||||||
|
Adding H to O+. Once again we will begin with the fundamental question: | ||||||||||
| ||||||||||
|
Since O+ has the same configuration as the neutral N atom, each of its three 2p orbitals is singly occupied. It therefore has a nominal valence of 3, answer (c). We thus expect to find three compounds in the HnO+ family: OH+, H2O+, and H3O+. The figure depicts both the bonding diagrams and the optimized structures: | ||||||||||
20.4 | 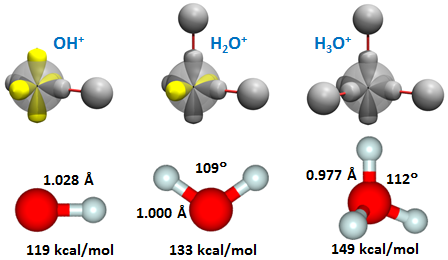
| |||||||||
|
Like all diatomic molecules, OH+ (the OH cation) is linear. The bond lengths of all of the cation compounds are longer than their neutral counterparts. The bonding diagram for H2O+ (the water cation) is similar to that of H2O, except that their is a singly occupied 2p orbital perpendicular to the plane containing the three nuclei. We expect a nominal bond angle of 90°. Like H2O, forces in H2O+ open up the angle, in this case to 109°. Finally, H3O+, which is known as hydronium, is expected to have a non-planar pyramidal structure with three equivalent bonds. The nominal bond angles are again 90°. The real angle is 112°, more than 20° larger than the nominal value but only 3° larger than the bond angle of H2O+. The bond energies are comparable to the values for the neutral and anion compounds. | ||||||||||
|
H3O+ and dative bonding. We built the HnO+ family by starting with O+ and recogizing that it could form three covalent bonds to H atoms. But H3O+ can also be formed by adding H+ to H2O via dative bonding. Remember that dative bonding occurs when one entity provides the pair of electrons that is shared between two entities. In this case, H+ has no electrons and an unoccupied 1s orbital. It interacts with the 2p2 pair of H2O to form hydronium. The animation below shows the formation of the bond. | ||||||||||
20.5 | 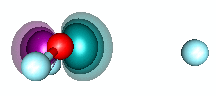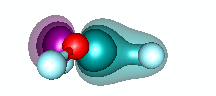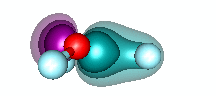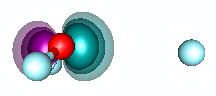
| |||||||||
|
The network of HnOp compounds. In this section we've learned about six compounds that can be constructed from one O atom in its neutral, cation, and anion charge states and various numbers of H atoms. The diagram below shows how these nine species are interrelated: | ||||||||||
20.6 | 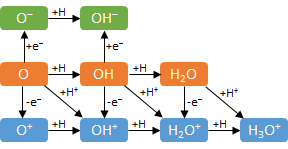
| |||||||||
|
The additions of H atoms moving from left to right along one of the three rows in the diagram are the sequential bond energies described above. But there are there other energy quantities represented in the graph as well. In Section 8 we looked at the ionization energies (IE) and electron affinities (EA) of atoms, including O. Since molecules can also be ionized or accept an electron, they also have IEs and EAs. The other quantity is called the proton affinity (PA). We'll briefly discuss each of these. | ||||||||||
|
IONIZATION ENERGIES. The diagram shows three cases where we can compute an IE: going from O to O+, from OH to OH+, and from H2O to H2O+. The three respective calculated values are for these IEs are 13.5, 13.0, and 12.6 eV. While there is a net change of more than 1 eV across the series, they remain fairly similar. That makes sense, since in each case the electron being removed is coming from a 2p2 pair. | ||||||||||
|
ELECTRON AFFINITIES. The diagrams shows two cases where we can compute an EA: going from O to O– and from OH to OH–. The respective calculated values for these EAs are 1.4 and 1.8 eV. Again, the values are comparable for a reason that makes sense: in both case an electron is being added to a single occupied 2p orbital. | ||||||||||
|
PROTON AFFINITY. The proton affinity is the energy gained by adding H+ to a neutral compound. The diagram shows three cases of this: going from O to OH+, from OH to H2O+, and from H2O to H3O+. The respective calculated values for these PAs are 120.6, 147.2, and 171.6 kcal/mol. In each case the H+2 pair, but it become increasingly favorable to do this as the number of neutral atoms added to O increases. | ||||||||||
|
Finally, the diagram from above has been modified below to indicate the more reactive compounds with singly occupied orbitals (red borders) and less reactive ones without singly occupied orbitals (black borders). The sequence in each row was completed when enough H atoms were added so that all of the singly occupied orbitals have formed a bond. We call these closed shell compounds. | ||||||||||
20.7 | 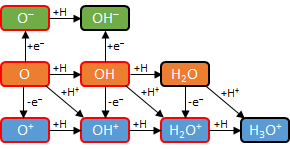
| |||||||||
|
Many of the H-containing compounds constructed from O, O+, or O– are important in chemistry. Water is one of the most important compounds of all. The hydroxyl radical is a reactive intermediate found, for example, in atmospheric chemistry. The hydronium ion is central in acid-base chemistry. The hydroxide anion can form salts with some metals. Even an oddball molecule like H2O+ shows up as an observed astromolecule. | ||||||||||
|
In the next section, we will look at the neutral H-containing compounds of an atom that is quite similar in some ways to O, sulfur (S). | ||||||||||
| Click on the link to proceed to the next section: | 21. HnS Compounds |
|---|---|
| MODULE TABLE OF CONTENTS | |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|