
| ||
|---|---|---|
| PREVIOUS SECTION | CURRENT SECTION | NEXT SECTION |
| 17. Summing Up | 18. Atom-by-Atom Approach | 19. HnO Compounds |
| TABLE OF CONTENTS FOR CHAPTER 3 | ||
|---|---|---|
| 18. Building Molecules Atom-by-Atom | ||
|
Overview and bonding summary. In Chapter 1 we learned about the nature of atoms, and then in Chapter 2 we took our first steps toward building molecules from them by focusing on diatomic molecules. We learned that there are a number of different types of chemical bonds. In almost every case of bonding we investigated, we found that the bond depends upon forming a new coupled pair of electrons with antiparallel spin or changing the disposition of an existing pair of electrons in some way. Nonpolar and polar covalent bonds as well as ionic bonds involve forming new bonding pairs. These bond pairs which may be polarized a lot (ionic bonds), partially (polar covalent bonds), or not at all (nonpolar covalent bonds). In dative bonds, an existing pair of electrons on one atoms becomes a shared pair on two atoms or an atom and an ion. Finally, recoupled pair bonds occur when an existing electron pair is disrupted by a new bonding partner that is able to couple its unpaired electron with one from the existing pair. | ||
|
Representations of atoms. Back in Chapter 1 we built models for atoms that show the occupations of valence s and p orbitals. Here is the original set: | ||
19.1 | 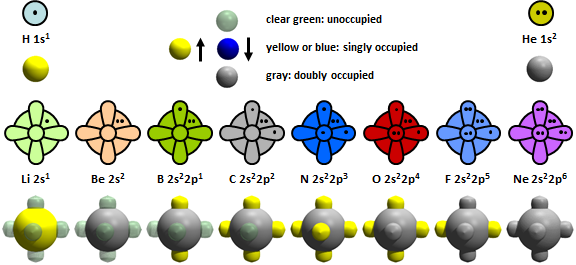
| |
|
In Chapter 2 we learned that some of these initial representations were inadequate. In particular, the discovery of recoupled pair bonding can occur in Be, B, and C demonstrated that the models for those atoms needed to be updated to reflect that their 2s2 pairs can be recoupled. Here is a better set of reprentations for H through Ne: | ||
19.2 | 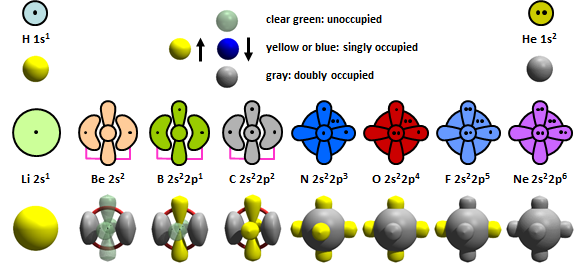
| |
|
Building up from one atom & understanding reactivity. Our approach in this chapter will be to start with one or two atoms and then add as many of another type of atom as we can. We are interested in how the atoms come together and what the structures look like. We're also interested in the strength of the various bonds. Finally, we're interesting in how we can gauge the reactivity of each compound and how it compares to other similar compounds. | ||
|
Reactivity is a measure of how likely it is for a chemical reaction to take place. It is very dependent on conditions such as temperature, what sources of energy are available, and what other compounds are present. For example, gasoline is very reactive toward oxygen, but it can be stored fairly safely in gas tanks as long as it is not exposed to sparks or open flame. | ||
|
In this chapter, we will focus on relative reactivity and try to understand why one compound would be more reactive than another. | ||
|
In the next section, we will begin our exploration of atom-by-atom construction of molecules by adding as many H atoms as we can to an oxygen atom. | ||
| Click on the link to proceed to the next section: | 19. HnO Compounds |
|---|---|
| MODULE TABLE OF CONTENTS | |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|