
| ||
|---|---|---|
| PREVIOUS SECTION | CURRENT SECTION | NEXT SECTION |
| 23. CHn Compounds | 24. More C Compounds | 25. HnO2 Compounds |
| 24. More C Compounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Compounds that contain carbon are called organic compounds because they are associated with living material. Here we will consider six small organic compounds that can be made from some of the CHn compounds we characterized in the previous section. The three intermediates of interest are the version of CH with three singly occupied orbitals, the version of CH2 with two singly occupied orbitals, and the CH3 radical, which are known as the methylidyne, methylene, and methyl radicals, respectively. We will examine each of these cases in turn. For reference, here are the 2D and 3D diagrams for each radical: | ||||||||||
24.1 | 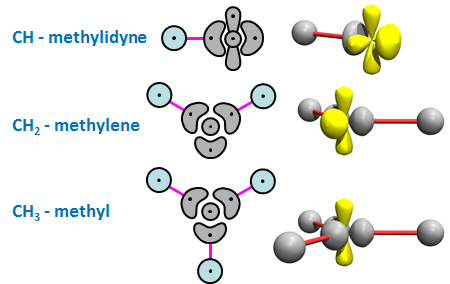
| |||||||||
|
CH radical as a starting point. With its lobe orbital aligned with the CH bond axis and two off-axis singly occupied 2p orbitals, CH resembles the N atom to a degree. Back in Section 16 we found that we could join two N atoms together with a triple bond. The bonds consisted of a sigma bond and two pi bonds. | ||||||||||
| ||||||||||
|
Since the singly occupied lobe orbital of CH is 180° away from the the CH bond pair, we would expect HCN to be linear, answer (c). HCN or hydrogen cyanide is indeed linear, as shown in the structure below. The formation of 2D and 3D bonding diagrams is also depicted. | ||||||||||
24.2 | 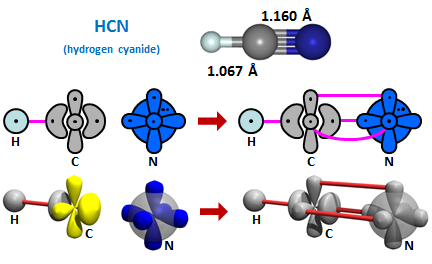
| |||||||||
|
Note that HCN will not form unless each of the three electron pairs is an antiparallel spin combination. The next thing we can do is to combine two CH radicals, just as we combined two N atoms, to form C2H2, which is also known as acetylene: | ||||||||||
24.3 | 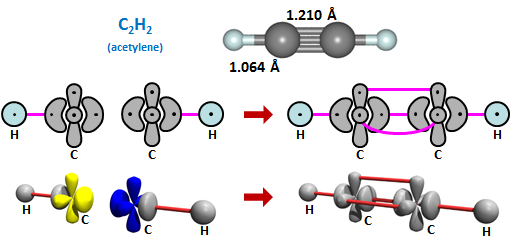
| |||||||||
|
Like HCN, acetylene is linear. This triple bonded form of carbon is found frequently in organic compounds, whether it is as the linear R1C≡CR2 or the R1C≡N motifs (where R1 and R2 can be varied considerably). | ||||||||||
|
CH2 radical as a starting point. CH2 has an in-plane lobe-like orbital and an out-of-plane 2p orbital and has nominal bond angles of 120°. One atom we can bond to it is O, as shown below: | ||||||||||
24.4 | 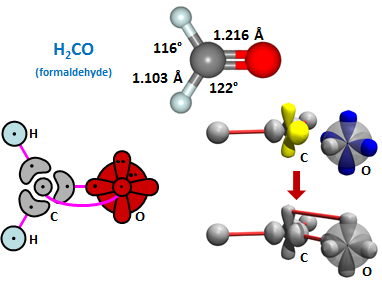
| |||||||||
|
Here we form a double bond between C and O, with one sigma bond and one pi bond. This forms H2CO or formaldehyde. Note that the bond angles are only slightly distorted from the nominal 120° bond angles. As with CH, we can assemble two CH2 radicals to form C2H4, also known as ethylene: | ||||||||||
12.5 | 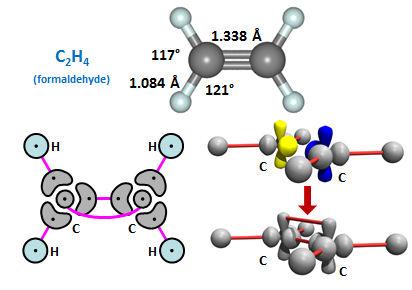
| |||||||||
|
The double bonded form of carbon is also found frequently in organic compounds. The two motifs here are both planar: R1R2C=CR3R4 and R1R2C=O (where any of the Ri substituents can be varied considerably). | ||||||||||
|
CH3 radical as a starting point. The methyl radical, CH3, is planar until a fourth bond is formed, when it becomes tetrahedral as in methane. Here we show replaced the fourth H with the hydroxyl radical, OH, to yield CH3OH or methanol: | ||||||||||
24.6 | 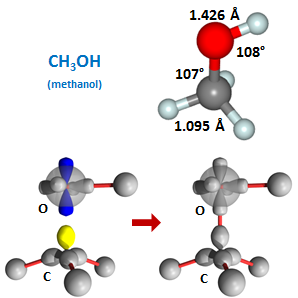
| |||||||||
|
The bond angles around the carbon are again only slightly distorted from the tetrahedral angles of 109.5° (and note that the C-O-H angle again opens up considerable from the nominal 90° angle). The last compound we will look at in this section is formed by combining two methyl radicals to yield C2H6, which is known as ethane: | ||||||||||
24.7 | 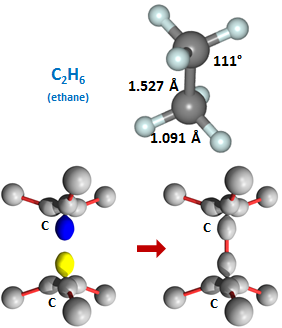
| |||||||||
|
An enormous number of other compounds can be formed by replacing any of the H atoms in either methanol or ethane with other groups or hydrocarbon chains. | ||||||||||
|
Summary. In this section we've barely dipped into the literally limitless combinations of carbon-containing compounds that are possible. We've seen three different motifs for CC or CX bonds (where X can be N, O, and other elements): triple, double, and single bonds. We saw how each of these motifs arises straightforwardly from one of the CHn compounds. | ||||||||||
|
In the next section, we will return to the oxygen, combining two O atoms to form O2 and then exploring the addition of H. | ||||||||||
| Click on the link to proceed to the next section: | 25. HnO2 Compounds |
|---|---|
| MODULE TABLE OF CONTENTS | |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|