
| ||
|---|---|---|
| PREVIOUS SECTION | CURRENT SECTION | NEXT SECTION |
| 22. BeHn Compounds | 23. CHn Compounds | 24. More C Compounds |
| 23. CHn Compounds | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In Section 15 we discovered something fascinating about the carbon atom: we can a H atom to it in two different ways to produced two very different forms of the CH radical. By way of review, we start with the 2D and 3D diagrams for the atom: | ||||||||||||
23.1 | 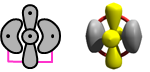
| |||||||||||
|
The carbon atom has four valence electrons. Two of this are in singly occupied 2p orbitals. The other are the coupled lobe pair that combines the character of the 2s2 pair and the unoccupied 2p orbital. We found that it is possible for H to form a covalent bond with one of the singly occupied 2p orbitals, but it can also form a recoupled pair bond with the lobe pair. Here are the two forms of CH we found: | ||||||||||||
23.2 | 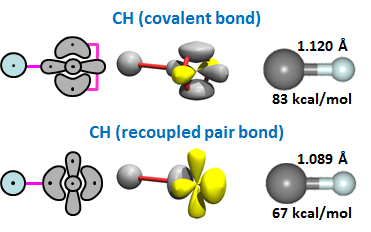
| 
| ||||||||||
|
The covalently bonding form has a stronger bond energy than the form with the recoupled pair, but only by 16 kcal/mol. Remember that the form of CH with the covalent bond has one singly occupied orbital available for further bonding (the 2p orbital), while the form of CH with recoupled pair bond has three singly occupied orbitals available for further bonding (two 2p orbitals and the outer lobe orbital left over from recoupling). | ||||||||||||
|
Adding the second H to CH (covalent bond form). We'll start by adding H to the more stable form of CH, the one with the covalent bond. | ||||||||||||
| ||||||||||||
|
In this case, the second H is being bonded to a singly occupied 2p orbital—just like the first H. The orbitals are 90° apart, just as they are in H2O, so the nominal bond angle is 90°, answer (d). Here is the structure of the first form of CH2, along with the 2D and 3D bonding diagrams: | ||||||||||||
23.3 | 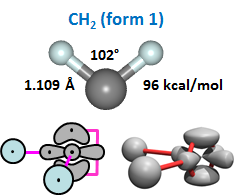
| |||||||||||
|
The optimal bond angle of CH2 form 1 is 102°, so the angle has opened up just the way the bond angle of H2O opens up. In fact, this for of CH2 is a lot like a water molecule. | ||||||||||||
|
Adding the second H to CH (recoupled pair bond form). Now we'll add H to the other form of CH, the one with the recoupled pair bond. This case is trickier to think about, because we have two choices: the H might forma a bond with the leftover lobe orbital, or it might forma a bond with one of the two singly occupied 2p orbitals. | ||||||||||||
| ||||||||||||
| ||||||||||||
|
In the first case, the 2p orbitals lie at 90° angles with the CH bond axis, so the nominal bond angle would also be 90°, answer (d). In the second case, the leftover lobe is 180° away from the one being used in the bond, so the nominal bond angle would be 180° like in BeH2, answer (a). Let's see what he real bond angle is: | ||||||||||||
23.4 | 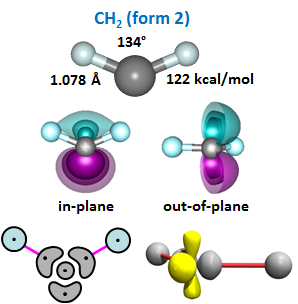
| |||||||||||
|
The bond angle is not close to either 90° or to 180°. It's closer to 120°. The figure also shows the two singly occupied orbitals. One of them, the out-of-plane orbital, is a 2p orbital. The other, the in-plane orbital, looks a little bit like a lobe orbital and a little bit like a 2p orbital. This form of CH2 has found a compromise: it's in the middle ground, partly bonding to the both types of orbital, yielding a bond angle of 134°. | ||||||||||||
|
The last part of Figure 23.4 shows the 2D and 2D bond diagrams we'll use for this form of CH2. Note that the representations for the two singly occupied orbitals have the characters of the actual orbitals: a 2p orbital and a lobe-like orbital. | ||||||||||||
|
Which form of CH2 is more stable? To determine this, we add the energy of the second bond to the energy of the first bond in both forms. The values of the bond energies are provided in Figures 23.3 and 23.4. For form 1, we have 83 + 96 = 179 kcal/mol. For form 2, we have 67 + 122 = 189 kcal/mol. Form 2 is more stable! The amount is small—just 10 kcal/mol—but the impact of this small advantage is enormous. The reason that form 2 is more stable is because its second bond is much stronger than the second bond in form 1, 26 kcal/mol stronger. This is why form 2 is the preferred form, and it is why we can add than two H atoms to C. There's a good chance that if the water-like, closed shell form of CH2 was more stable than the form with two singly occupied orbitals, life itself would not exist. The vast majority of organic chemistry and biochemistry is possible because carbon has a valence greater than two. | ||||||||||||
|
Adding a third H to CH2 (form 2). We once again have a case where there are two types of orbitals that H could bond to in CH2, the in-plane and the out-of-plane orbitals. Here, however, we see a preference, rather than a compromise: | ||||||||||||
23.5 | 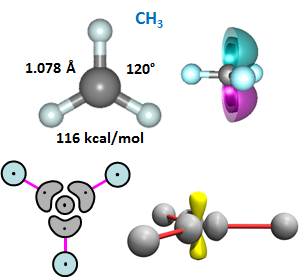
| |||||||||||
|
The third H bonds to the in-plane, lobe-like orbital and yields three equivalent CH bond lengths and bond angles of 120°. There is still a singly occupied orbital, and it is a 2p orbital that is perpendicular to the plane containing the four nuclei. The 2D and 3D bonding diagrams are shown. | ||||||||||||
|
Adding a fourth H to CH3. When we add the fourth H, we see the other three H atoms move away it. Here's an animation showing the formation of CH4: | ||||||||||||
23.6 | 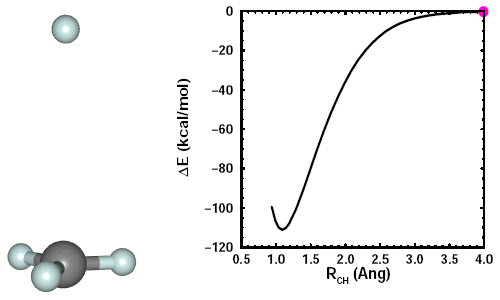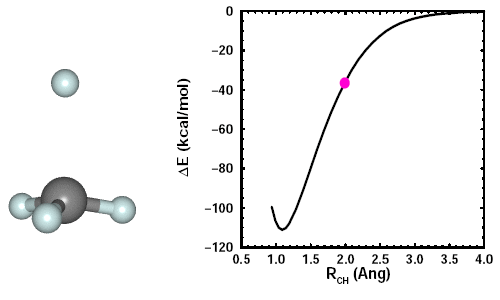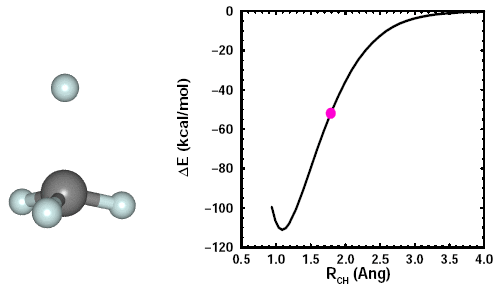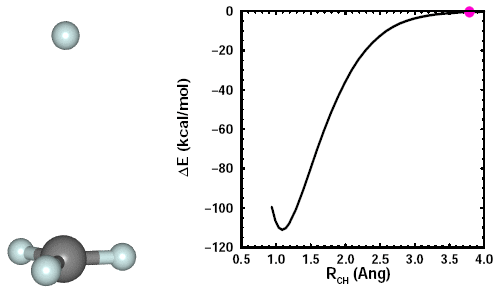
| |||||||||||
|
The CH3 maintains planarity until the fourth CH bond starts to form. It then adopts a tetrahedral arrangement of the four CH bonds, which minimizes the bond-bond repulsions. The figure shows the structure of CH4, also known as methane, and its 2D and 3D bonding diagrams: | ||||||||||||
23.7 | 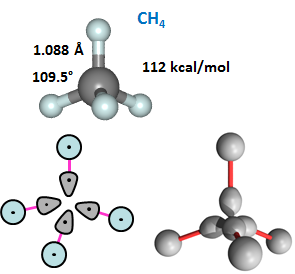
| |||||||||||
|
In this case, the 2D diagram is not very successful conveying the 3D character of of CH4. | ||||||||||||
|
Adding a fifth H to CH4. If we attempt to add one more H to CH4, we find that we cannot form CH5. | ||||||||||||
|
In the next section, we will assemble some common compounds containing carbon that shows the usefulness of the bonding diagrams. | ||||||||||||
| Click on the link to proceed to the next section: | 24. More C Compounds |
|---|---|
| MODULE TABLE OF CONTENTS | |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|