
| ||
|---|---|---|
| PREVIOUS SECTION | CURRENT SECTION | NEXT SECTION |
| 21. HnS Compounds | 22. BeHn Compounds | 23. CHn Compounds |
| 22. BeHn Compounds | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
We will now return to the Be atom. Back in Section 14 we discovered that one H will form a bond to Be even though there are no singly occupied orbitals available on Be for making a covalent or ionic bond. That means that if we naively ask the question... | |||||||||||||||||
| |||||||||||||||||
|
... the answer is 0, response (a), because it has no singly occupied orbitals. We already know that this answer is not correct for Be, because BeH was found to form via recoupled pair bonding. Let's briefly review what that means. | |||||||||||||||||
|
We tried to form bonds between H and a number of electron pairs—in He, Ne, F, and most recently with O in Section 19—and found only repulsive interactions in those cases. However, we did find three cases where bonds do form with electron pairs—in Be, B, and C—because in those elements H is able to break apart the coupling between their lobe pairs in order to form a bond. We called this recoupled pair bonding. Here are 2D and 3D bonding diagrams for BeH: | |||||||||||||||||
22.1 | 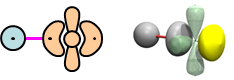
| ||||||||||||||||
|
We noted that BeH has a singly occupied orbital, which is the lobe orbital that is leftover after recoupling. Based on this observation, try to answer the following question: | |||||||||||||||||
| |||||||||||||||||
|
Be has two valence electrons. H was able to form a bond with one of the electrons in the lobe pair, and it's reasonable to think that H can form a second bond with the leftover lobe orbital electron. That would give Be a valence of 2, answer (b). If this is true, it means that Be has an effective valence of 2. | |||||||||||||||||
| |||||||||||||||||
|
The lobe orbitals are 180° apart, so we might expect a bond angle of 180°, answer (a). Here are calculated structures and successive Be-H bond energies for BeH and BeH2: | 
| ||||||||||||||||
22.3 | 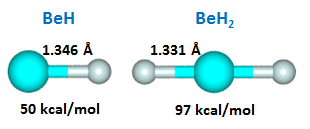
| ||||||||||||||||
|
The expectation that BeH2 might be linear proved to be correct. It's noteworthy that the first bond in BeH is much weaker than the seccond bond in BeH2. They differ by nearly a factor of two. The bonds are also shorter in BeH2. Let's look at the four orbitals in the two bond pairs: | |||||||||||||||||
22.4 | 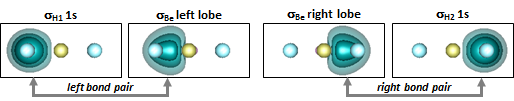
| ||||||||||||||||
|
The orbitals are consistent with what we saw in Section 14. The left and right lobe orbitals on Be that are each bonded to H in BeH2 still resemble the atomic Be orbitals depicted in Figure 14.4, although they have delocalized significantly onto the H atoms. | |||||||||||||||||
|
We can use the following bonding diagrams for BeH2: | |||||||||||||||||
22.5 | 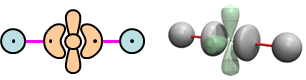
| ||||||||||||||||
|
We've simply coupled the second H atom's 1s orbital to the singly occupied outer lobe orbital of BeH in Figure 22.1. | |||||||||||||||||
|
Since the second bond in BeH2 involves using the electron left over from recoupling the lobe pair on Be, there's a name for the pair of bonds: a recoupled pair bond dyad. The second bond is always much stronger than the first bond, because there is a cost to breaking up the pair and because the leftover electron makes the first bond weaker. These two bonds are coupled in a way that two covalent bonds are not (in H2O and H2S, for example): their origin from a lobe pair means that they are ideally 180° apart. We will now add this type of bonding to the bonding list: | |||||||||||||||||
| |||||||||||||||||
|
Note that although the reduction in bond length between BeH and BeH2 is small, it is a well-defined characteristic of recoupled pair bond dyads. | |||||||||||||||||
|
We cannot add a third H to BeH2. | |||||||||||||||||
|
In the next section, we will explore adding H to C. | |||||||||||||||||
| Click on the link to proceed to the next section: | 23. CHn Compounds |
|---|---|
| MODULE TABLE OF CONTENTS | |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|