
| ||
|---|---|---|
| PREVIOUS SECTION | CURRENT SECTION | NEXT SECTION |
| 20. HnO+/HnO– | 21. HnS Compounds | 22. BeHn Compounds |
| 21. HnS Compounds | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The sulfur atom resembles the oxygen atom: they both have an s2p4 valence shell. Sulfur atom has 8 more electrons because its n=2 shell is filled. It's valence electrons are in the n=3 shell, 3s23p4. We can use the same representations for S as we used for O: | ||||||||||||
21.1 | 
| |||||||||||
|
All we've changed in the 2D diagram is the color (which is completely arbitrary). The 3D diagram is the same as the one for O. | ||||||||||||
| ||||||||||||
|
Since S is similar to O and has two singly occupied 3p orbitals, it also has a nominal valence of 2, answer (b). We expect to be able to form SH and H2S. | ||||||||||||
| ||||||||||||
|
Analogous to O, S has two singly occupied 3p orbitals that are 90° apart, so the nominal bond angle of H2S is also 90°, answer (d). The bond diagrams and calculated structures for SH and H2S are shown below. The structures of OH and H2O are included for comparison. | ||||||||||||
21.2 | 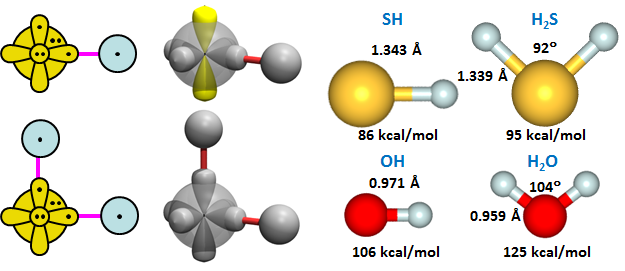
| |||||||||||
|
We can make a couple basic observations. The SH bond lengths in both compounds are more than 0.3 Å longer than the OH bond lengths, which is a consequence of the 8 extra electrons in the core of S. The bonds are also decidedly weaker, by 20-25%. The bond angle of H2S is very close to the nominal bond angle of 90°. | ||||||||||||
|
In Section 19 we argued that the bond angle of H2O opens up by more than 10° from the nominal bond angle mostly due to repulsion between the bond pairs. The bond angle of H2O opens up very little from the nominal bond angle, which implies that there is less repulsion between the bond pairs of H2S than H2O. Let's compare the bonding pair orbitals of SH vs OH and then H2S vs H2O to see if this logic is supported by the facts. We'll start with the diatomics and look at the orbitals at the minimum separation: | 
| |||||||||||
21.3 | 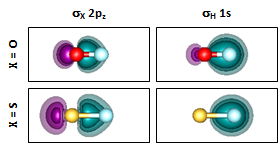
| |||||||||||
|
Note where the H contours cross the bond axis in the two compounds. They almost reach the O nucleus but are much closer to the midpoint of the bond in SH. There is therefore less polarization of the H 1s orbital in SH than in OH. Let's see if this is also true in H2O and H2S: | ||||||||||||
21.4 | 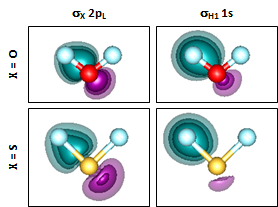
| |||||||||||
|
Here we are looking at just one set of the bond orbital for H2O and H2S, on the left side. The polarization is very similar in H2O and H2S as in their corresponding diatomic. The more each H 1s orbital is polarized toward O or S, the more it will tend to overlap the other H 1s orbital, which results in greater repulsion. We can see the interference even better if we look at both orbitals superimposed together: | ||||||||||||
21.5 | 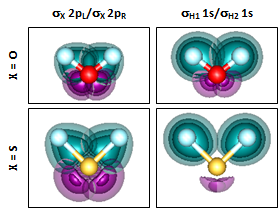
| |||||||||||
|
We can now see quite clearly that the overlap is much worse in H2O than it is in H2S. Part of it is because the polarization toward O is more pronounced than the polarization toward S, but we can also see that the longer bond lengths of H2S mean that the H atoms are farther apart in H2S than in H2O and thus the overlap is reduced. The orbitals demonstrate quite well why the nominal bond angle prediction of 90° is so much closer to the real bond angle for H2S than it is for H2O. | ||||||||||||
|
In the next section, we will look at adding H to the Be atom. | ||||||||||||
| Click on the link to proceed to the next section: | 22. BeHn Compounds |
|---|---|
| MODULE TABLE OF CONTENTS | |


|
| Copyright 2011-2017 University of Illinois. All rights reserved. |
|---|